
Features
Diseases
Top Crop Summit
Top Cop Summit series: Bacterial leaf streak of wheat
March 26, 2024 By Bruce Barker
 Symptoms of bacterial leaf strip show up as water-soaked streaks with bacterial ooze. Photo courtesy of Kelly Turkington.
Symptoms of bacterial leaf strip show up as water-soaked streaks with bacterial ooze. Photo courtesy of Kelly Turkington. Valentina Anastasini is a MSc student in cereal and flax pathology at the University of Saskatchewan (U of S) under the supervision of Drs. Fleitas and Kutcher. At the Top Crop Summit on Feb. 27-28 in Saskatoon, she presented recent research findings looking at bacterial leaf streak.
Bacterial leaf streak (BLS) is an emerging foliar disease reported worldwide that has become prevalent in the Canadian Prairies within the last decade. The pathogen has a wide range of hosts. BLS of wheat is caused by the bacterium Xanthomonas translucens pv. undulosa. It may also be associated with barley, but another related bacterium Xanthomonas translucens pv. translucens is more problematic in barley.
The disease is caused by a seed-borne bacterium and has a direct impact on yield, grain quality, and reduces seedling emergence. It may survive in crop debris to infect subsequent cereal crops. Wind-driven raindrops can spread the bacterium. Infection occurs through plant wounds, possibly from wind-driven debris or hail damage, or through naturally occurring stomata leaf pores.
Weather conditions conducive to disease development include moist, humid conditions and warm temperatures between 15 to 30°C.
Management of BLS is difficult since there are no effective chemical treatments that entirely control the pathogen. Fungicides do not work because fungicides are for fungal diseases and BLS is caused by bacteria.
Early leaf infections show up as translucent, water-soaked streaks, which differentiates it from other leaf spot diseases. Under humid conditions, bacterial ooze may show up. Streaked lesions can develop on the leaves. The loss of photosynthetic power can result in 30 to 40 per cent yield loss. If the disease moves to the plant head, glumes can develop dark-purple streaks resulting in ‘Black Chaff’ disease, and further quality losses.
An ongoing project at the U of S is researching integrated management tools for BLS disease of wheat in Canada. One aspect of the project is to evaluate 500 accessions of Triticum spp. for resistance to BLS under field and controlled conditions. Of those 500 accessions, 95 were Saskatchewan varieties, 105 elite lines in breeding programs and another 300 from a diverse panel of accessions.
Field screening of the varieties took place at Outlook, Sask., and Lacombe, Alta., under irrigation. Two check varieties were used for comparison: Alondra (susceptible) and Pavon (resistant). The accessions were sprayed at the flag leaf and heading stage with an isolate of the pathogen. Evaluation of disease severity took place 15 days after inoculation at heading, late milk and early dough stages.
In the greenhouse, spray inoculation occurred at the third leaf stage followed by the plants placed for two days in a mist chamber. Disease severity was assessed 15 days after inoculation.
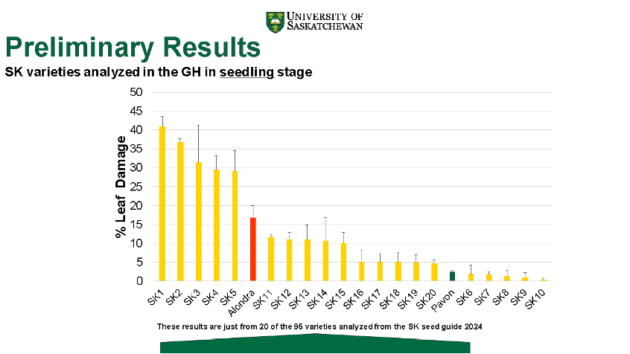
Resistance to the pathogen varied across accessions. An example of the variability was seen in preliminary assessments of 20 of 95 Saskatchewan varieties analyzed. Five of 20 showed percentage leaf damage as low or lower than the known resistant variety Pavon. Others had much higher leaf damage than the susceptible Alondra.
Future BLS work includes:
- Continue evaluation of wheat accessions in the greenhouse
- Field evaluation of the germplasm set
- Early detection in seed (qPCR)
- Assessing seed to seedling transmission of Xt causing BLS of cereals to establish inoculum thresholds
- Determine seed to seedling transmission rate of Xt under field conditions to establish risk threshold levels
- Effect of temperature and seed treatments on multiplication of Xt and symptom expression in seed with varying infection levels
Farmers and agronomists are encouraged to scout for the disease and keep field records to help track the disease on their fields. Test seed for infection and avoid using infected seed. More research is required to understand the impact of seed treatments.