Features
Diseases
Pulses
Evaluating root rot in pulses
Exploring Aphanomyces research and the role its accomplices play in driving disease.
June 3, 2020 By Presented by Syama Chatterton, Agriculture and Agri-Food Canada, at the Top Crop Manager Plant Health Summit, Feb 25-26, 2020.

The root rot problem really exploded in about 2011-2012. Most pathologists were under the assumption that it was Fusarium root rot. But we saw that a lot of things didn’t add up, particularly with the symptomology.
Fusarium root rot tends to cause blackening of the tap root and it doesn’t usually extend into the lateral roots. Instead, in the field, we were seeing roots that were totally decayed, often with complete loss of the lateral roots along with honey-brown and black discolouration.
We were also seeing widespread damage in some fields. Generally, Fusarium root rot doesn’t do this, so we knew that there was something else going on. What we found was an unexpected surprise – the pathogen Aphanomyces euteiches. It causes very distinct symptoms on roots and a honey-brown discolouration. The most telltale symptom is pinching of the epicotyl, and then often what happens is Fusarium comes in afterwards, really making symptoms difficult to diagnose.
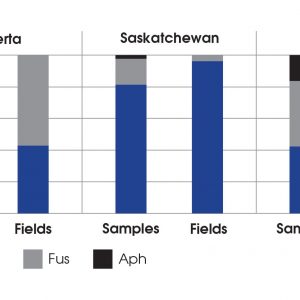
Aphanomyces was reported for the first time in Saskatchewan in 2012 and in Alberta in 2013. Large-scale surveys across Alberta, Saskatchewan, and Manitoba were conducted to look at the prevalence of Aphanomyces root rot, and to also look at what other pathogens were involved. The surveys from 2014 to 2017 found that Aphanomyces was widespread across the Prairies. The hypothesis is that it is a native pathogen; it’s in the soil. After extensive production of pea and lentils – fields with a history of 20 to 25 years, even with a pea or lentil once every five years – the inoculum increases above threshold causing these pulse crops to get the disease.
In Alberta over the past five years, prevalence of Aphanomyces was between 40 to 50 per cent of the fields surveyed. The survey period had really wet years and really dry years, and for the most part, prevalence and incidence went up and down with moisture. In Alberta over the last few dry years, about 30 to 40 per cent of fields were positive. Unfortunately in Saskatchewan, there were peaks in 2015 and 2016 around 65 to 70 per cent Aphanomyces prevalence, down a little bit in 2017 and 2018 at about 40 per cent prevalence, and then, for some reason, in 2019 it was the worst that we have seen yet with about 90 per cent of fields showing positive symptoms. Precipitation in 2019 was a weird year. The average precipitation wasn’t really reflective of what happened in the season, because it was dry in the spring, and plants were stressed. Then with rain later in the spring, it got so wet that Aphanomyces was able to infect in July. Because the plants were already stressed, the disease really took off.
Root rot is a complex. Aphanomyces is our A-list pathogen, but there are some other pathogens that are also contributing to the problem. There are Pythium species and Rhizoctonia species, and many different Fusarium species. For Alberta, between 10 and 90 per cent of the fields are positive for these pathogens. Saskatchewan and Manitoba are virtually the same story.
The other issue is that Fusarium is also a problem on its own. Some fields only have Fusarium root rot, some fields have Aphanomyces plus Fusarium root rot, and a few fields have only Aphanomyces. In Alberta, Aphanomyces is almost always occurring with Fusarium. In Saskatchewan there is more of a balance of 50/50 Fusarium and Aphanomyces.
We tested pea and lentil in the greenhouse and found they are equally susceptible to Aphanomyces. The symptoms on lentils are virtually the same as they are on peas. But in the field, particularly in dry years, lentils seem to be less impacted.
Lab research
My lab research is conducted in the context of current management recommendations. A big portion of my research that I started four or five years ago is trying to come up with a risk quantification system so we can build some sort of decision support system based on soil testing.
The amount of inoculum that is required to cause disease is the foundation of any sort of risk quantification system. In order to do that, we are trying to measure the oospores in soil, which are the thick-walled resting spores. This is where we’ve run into some challenges. The thick cell walls means they don’t like to be cracked open so that we can test how much DNA is present.
In our research, we looked at the number of oospores per gram (oospores/g) of soil. At low levels, the pea plants were pretty healthy, but at 100 the plants looked very diseased. One hundred oospores isn’t good news because that’s a very low threshold level.
We also found different threshold levels for different soil types. Clay loam had the highest threshold level at 275 +/– 36.5 oospores/g, which is not what we were expecting, because we often see that disease is worse in these heavy clay fields. I think it has something to do with the water-holding capacity of the clay: it holds more water, so even though the threshold dose is higher, it tends to stay wetter for longer. Loam (98.8 +/– 42.9), Sandy Loam (81.3 +/– 76.7) and Silty Loam (44.3 +/– 38.3) had lower threshold levels.
The other research we are working on is using DNA tests to quantify the pathogen level and determine what the risk is. We compared measured oospores using DNA testing versus actual oospores/g of soil. The correlation was pretty good for sandy loam soils, less so for silty loam, but not very good for clay loam soils. For example, for clay loam, the DNA test is telling us there are about 10 oospores but the actual number of oospores is 100. This makes it difficult to test soils that are just at or below that threshold.
We took this comparison to the field and compared the same soils in the greenhouse with DNA testing. In the field soils, we find an average percent incidence of Aphanomyces of about 80 per cent. Our average disease severity is 4.2, and estimated about 1,000 oospores per gram of soil. In the greenhouse, we had very similar results.
But when we look at two different types of DNA testing methods, the DNA measurements were only detecting about 50 per cent of soils positive for Aphanomyces. The two DNA methods estimated 281 to 313 oospores/g of soil, but there was a huge range from 7.6 to 2,300 oospores/g. It’s the number of false negatives that are coming back from the DNA tests that is a challenge, and we’re working really hard on trying to improve that.
The other problem is that we’re underestimating the amount of Aphanomyces with DNA testing because of those multiple pathogens that are interacting together. So, we are also working on trying to make a multiple pathogen detection system, both for Aphanomyces and Fusarium, and that gets a little bit complicated, although I think we’re making progress.
Seed treatment trials for Aphanomyces
We’re doing field trials to assess our current recommendations to growers. The first is to consider using a seed treatment that targets the root rot complex. It wasn’t really clear which ones would be effective against Aphanomyces or whether there are any that are effective.
We tried a number of different seed treatments – some of them confidential – so I can’t the share the trade names. We also tried trifluralin pre-seed because there had been some reports of literature in the early ’90s that it had some effect on Aphanomyces root rot. The problem is that we couldn’t incorporate trifluralin in no-till fields so we were just applying it to a heavy crop residue. We also tried Phostrol as a foliar spray to the seedlings. There had been some previous research out of Washington that had shown this might be effective. Long story short, there was no effect of most treatments over four years at four locations.
Crop studies
The other recommendation we are looking at is whether a six-to-eight-year rotation away from peas and lentils is necessary because it is a bit of a guess based on what is in the literature. We wanted to see if that recommendation was different under Prairie conditions. And, are there other pulses that you can grow in these infested fields without increasing your risk?
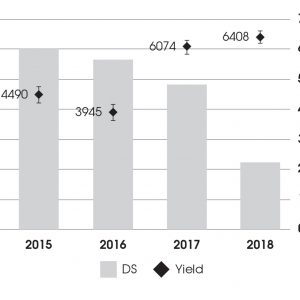
We looked at infected fields to see how long the rotation has to be out of peas before they can be rotated back into the field. One of those fields was at Red Deer in the Black soil zone. The last time peas were grown on this field was in 2011. In 2015 and 2016, there were still fairly high disease severities of around 6 on a scale of 0 to 7. Disease severity starts to come down in 2017, and then was quite low in 2018 at just above 2. By 2017 and 2018 there were some fantastic yields at this site around 95 bushels per acre (6,408 kg/ha). As disease severity was going down, yield was coming up. This indicates that maybe the six to eight year rotation recommendation is correct.
Red Deer – last pea crop, 2011
Rain (mm) 215 239 176 190
(LTA) 282 -25 -1 -64 -50
Contrast that to a site in southern Alberta at Taber. The last pea crop was in 2014, but over the past five years there hasn’t been a reduction in the disease severity – still between 4 and 6. The last three years have been very dry, but we haven’t seen the disease severity go down. The yields are much lower than what we saw up at that Red Deer site, as well.
The other thing that we looked at in Taber is how the pathogen composition is changing over time. Particularly we wanted to see what was happening with Aphanomyces and Fusarium. We took root samples in June, and the Aphanomyces levels were quite high on the roots, but the Fusarium levels were quite low. In July, the Aphanomyces levels on the roots were quite low, and the Fusarium levels went up. This shows part of the issue with surveying is that a lot of surveys are done around flowering in mid-July and we’re missing Aphanomyces. I think that’s another reason why we saw Aphanomyces explode over the Prairies. A good survey time for Aphanomyces is in June about six weeks after planting if you’ve had a good rainfall.
A new rotation study was started in 2018. Each site has a different break of one to five years since the last pea crop depending on when the producer last grew peas. In these rotation trials, cereal and canola were included as standard rotational crops. The trial gives us one- to eight-year breaks between a pea crop, with or without an alternate pulse crop in the rotation. The alternate pulse crop at Swift Current and Lethbridge was chickpea, fababean at Saskatoon and Lacombe, and soybean at Redvers, Brooks, and Morden.
The roots of peas and the alternate pulse crops were assessed for their pathogen levels. We also wanted to see how the soil inoculum potential changes with the different rotation lengths and different pulse crops. Soybean tells the best story with no Aphanomyces and even very, very low Fusarium levels on roots. But peas had very high levels of Aphanomyces, so, I think soybean can be a very good alternate crop to pea. With fababean the picture becomes a little less clear because at the Lacombe site, the disease levels had really come down in 2019. In 2020 we’re going to add peas back into the rotation and see how quickly the inoculum builds up. At the Saskatoon fababean site, the Aphanomyces root rot levels were fairly low on pea, but there were higher levels of Fusarium colonization on the roots. On fababean, Aphanomyces colonization was very low, and it isn’t as favourable of a host to Fusarium species either.
For chickpea, we saw very low root rot at Taber and Swift Current with Aphanomyces or Fusarium. At Taber, Aphanomyces colonization is higher on pea than chickpea, but Fusarium levels are much higher on pea. At this stage in the research, it appears that soybean and fababean could be good pulse crop options for managing Aphanomyces, and we’re still waiting to see if chickpeas might be a good option as well.
Differences in crop and variety reaction to Fusarium spp.
We also did some pathogenicity tests with F. solani and F. avenaceum on soybeans, fababeans, chickpea, red lentils, and Meadow pea. The first thing to note is that soybean, CDC Consul chickpea, and red lentil HAD pretty good emergence, even in the presence of F. solani. The disease severities for these were also all fairly low. The other chickpea varieties, Cory and Leader, and Snowbird fababean had lower emergence levels. Interestingly, CDC Orion did not even emerge when F. solani was present. Clearly, with chickpeas and fababean, a seed treatment should be used.
With F. avenaceum, there was a similar story. The soybeans had very good emergence with very little disease severity. The fababeans had poor emergence and fairly high disease severity, but it’s interesting that we see this in the greenhouse, but not out in the field. Chickpeas and red lentils also had poorer emergence but the green lentil variety Impower had good emergence and low disease severity. This shows the complications of all these different pathogens and how they work together and the importance of seed treatments for targeting Fusarium species.
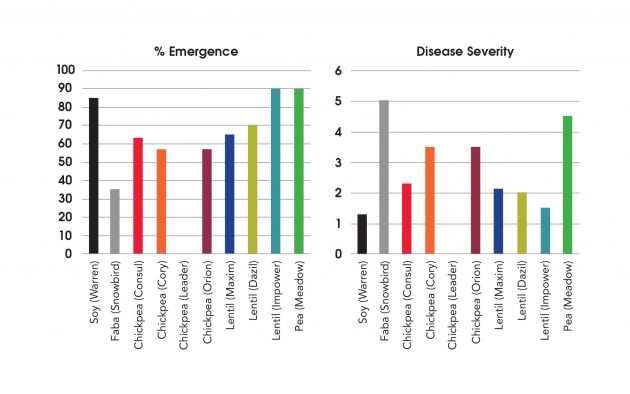
Virulence of Fusarium avenaceum to pulse crops
Biofumigation with cover crops
Brassica cover crops have been shown to have great biofumigation potential in the greenhouse, except for canola because the biofumigants have been bred out. Biofumigation is not like a seed treatment where it only works for four weeks. The cover crop could reduce oospore levels and bring that threshold down. In our field trials, we are looking at mustards and some other Brassica crops. Originally we tried two years of shoulder season cover crops planted after harvest in late summer/early fall. But the weather was either very dry or it snowed in September so we didn’t achieve a very competitive cover crop. As a result, the whole project was revamped and we’re looking at a full season cover crop to see if it works, and then we’ll figure out how to fit it into a cropping system. The cover crops being investigated are oats, rye, two mustards, fababean, clover and five different blends of Brassica crops that include mustards, tillage radish, Dwarf Essex rape, forage collards, forage rape, turnip rape and forage kale.
The cover crops are terminated as green manure with a flail mower. The biomass is removed, weighed and put back on the field, where one-half of the plot is tilled. Pea will be seeded into this cover crop footprint in 2020 and differences in root disease will be assessed. We are also trying to quantify the oospore numbers in the soil before and after cover crops, with and without tillage. Then we’ll repeat it so we have two years of data.
Another research project is looking at using legumes in a cover crop mix since this is becoming more popular with farmers. We planted vetches, clovers, lupins and peas in the field, and tested them for presence of Aphanomyces and disease severity. The vetches were very susceptible to Aphanomyces. Some clovers (Crimson, Yellow blossom, Persian) didn’t get a lot of disease, but they can support Aphanomyces. Other clovers (White Dutch, Red, Subterranean, Berseem) didn’t get the disease at all, and they didn’t support Aphanomyces colonization. Lupins didn’t support Aphanomyces either.
Liming as a management tool
Liming is a potential tool because calcium is very important in preventing oospores from germinating into zoospores. It’s very widely used in the U.S. in sugar beets to manage Aphanomyces root rot. Here, it might have potential for sugar beets or in systems where tillage can incorporate the lime. Liming may also have an impact for about five years, and it can help prevent clubroot of canola.
We did a greenhouse trial with soil from our Taber site and mixed different lime products at four different rates. Hydrated lime reduced disease severity with decreasing severity as the rate increased. Quick lime and ZeroG lime weren’t as effective, so there may be differences in the calcium availability of the products.
There was also an increase in root weight as the hydrated lime rate increased. Quick lime had some increase in root weight with increasing lime rates, but ZeroG did not have a significant increase. Our next step is to try and take this out into field trials and see if we have the same results as in the greenhouse.