
Features
Diseases
Stacking FHB resistance genes
Research on a recently discovered Fusarium toxin aims to improve resistance to this devastating disease.
April 4, 2023 By Carolyn King
 On the left is a typical Fusarium graminearum strain that produces gramillin infecting barley; on the right is a mutant strain that no longer produces gramillin and is no longer able to cause as much FHB. Thus, gramillin is an important factor in producing FHB in barley.
Photo courtesy of Whynn Bosnich, AAFC.
On the left is a typical Fusarium graminearum strain that produces gramillin infecting barley; on the right is a mutant strain that no longer produces gramillin and is no longer able to cause as much FHB. Thus, gramillin is an important factor in producing FHB in barley.
Photo courtesy of Whynn Bosnich, AAFC.
Fusarium graminearum, the main fungus causing fusarium head blight (FHB), is a wily enemy with multiple tactics for attacking plants. Those tactics include producing various toxins to facilitate its infection of a host. Now, a research project is looking into a recently discovered fusarium toxin in order to help breeders improve FHB resistance in their barley varieties.
Down the road, this research path has potential benefits for further improving FHB resistance and for fighting other crop diseases as well.
A flexible foe
Elizabeth Brauer, a research scientist in cereal phenomics with Agriculture and Agri-Food Canada (AAFC), is leading this project to advance FHB resistance. She outlines why it is so challenging to develop cereal varieties with strong resistance to FHB.
“Fusarium is a very flexible fungus in terms of its strategies for infection. It seems to have different modes of infection depending on which host it is in. As a result, the discoveries we make in one host might not necessarily apply to other hosts.
“Also, plant resistance to the fungus is governed by minor effect genes – many genes which each have a very subtle impact on the plant’s overall resistance to the fungus. Tracking and finding those small, subtle changes out in the field is really challenging and requires a lot of repetition.
“Another reason is that the fungus really responds to environmental fluctuations. How severe the infection will be is driven by things like temperature and humidity. So it can be difficult to monitor trends across different environments, for example.
“And then the other factor is that the infection cycle for fusarium head blight takes the whole life cycle of the plant. So we have to take more time to find the symptoms and measure the disease resistance or susceptibility.”
Discovering a new toxin
Brauer was part of the group that made the toxin discovery, which was published in 2018. “Linda Harris and Barbara Blackwell and both of their teams at AAFC in Ottawa co-discovered this. They were looking at host-specific gene expression; in other words, what kind of genes F. graminearum is turning on in corn versus wheat versus barley,” she explains.
The researchers noticed a cluster of fusarium genes that is expressed during the fungus’s infection on barley and on corn, but not on wheat. To investigate this, they ‘knocked out’, or eliminated the expression of some of these genes, and then looked at how that affected which compounds were produced by the fungus.
Through this method, they were able to identify a fusarium gene that makes two small molecules, which they called gramillin A and B. Adilah Bahadoor, who was then a post-doctoral researcher in Blackwell’s lab, figured out how to purify gramillin A and B, and determined their molecular structure.
“The gramillins are secondary metabolites. Secondary metabolites are small molecules that are not essential for the fungus’s basic growth and development, but they are helpful to the fungus in different environments,” Brauer explains. “In the case of the gramillins, they are produced specifically during infection.”
At the time, Brauer was a post-doctoral researcher working on plant genes involved in resistance to F. graminearum in Gopal Subramaniam’s group at AAFC-Ottawa. She was really intrigued by the discovery and asked Bahadoor if she could conduct some tests with gramillin, the purified mixture of the two gramillins.
“I was lucky enough that Adilah gave me a very small amount of it. When I injected it into plants, I found that it was an extremely potent toxin on plants. It killed them faster than any of the other stresses that I have ever imposed on plants in my career.” Even a small dose of gramillin could kill plant cells within hours.
This exciting finding prompted Brauer to work with Harris’s team on further testing. They established that these gramillins are ‘virulence factors’, meaning that they promote infection by the fungus. They don’t have any other function in the fungus’s growth.
“Interestingly, these virulence factors promote fusarium’s infection in corn but not in wheat,” she says. “Wheat was totally resistant to the toxic effects of gramillin.”
Gramillin and barley
Brauer, who now heads her own research group at AAFC-Ottawa, has continued to work on gramillin, with the overall goal of translating the basic discovery into something that is useful for farmers.
“The first thing we did was to treat lots of different organisms with the purified gramillin, including grasses and dicots, which are another lineage of plants, and fungi, bacteria and human cells,” she says.
“We found that the toxicity of the gramillins was really specific to plants. So, based on our preliminary analysis, gramillin will not likely be another highly regulated Fusarium mycotoxin like DON [deoxynivalenol, which is toxic to humans and animals as well as plants].”
These tests also showed that barley, like corn, is very sensitive to gramillin, and that F. graminearum uses gramillin in its attack on barley. In addition, the tests showed some variation in gramillin resistance/susceptibility across different barley cultivars.
The findings suggested that gramillin is essential to producing the fungus’s full infection in barley and that a barley variety’s ability to resist gramillin may give it resistance to FHB.
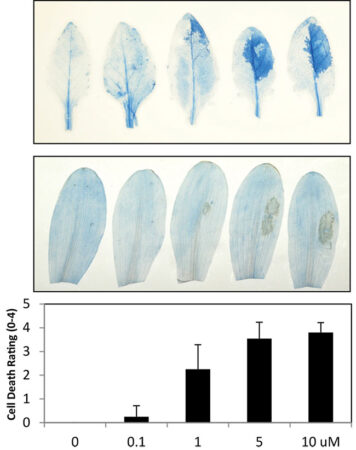
Brauer’s tests showed that gramillin is toxic to certain plants such as Arabidopsis (a relative of canola, top) and corn (bottom); dead cells show up as blue or beige on these images.
Photo courtesy ofElizabeth Brauer, AAFC.
Delving deeper
With funding from the Saskatchewan Barley Development Commission and the Manitoba Crop Alliance, Brauer and her team started their new gramillin/barley project in 2021.
This project is digging into the mechanisms and genes involved in gramillin resistance in barley and working to develop tools to help Canadian barley breeders bring gramillin resistance into their varieties.
“One of the project’s goals is to figure out why one variety of barley is more resistant to gramillin than another or why one crop type is more resistant than another, and how that relates to Fusarium infection. In other words, what is gramillin actually doing to allow the fungus to overtake the plant’s resistance?” she says.
“We have been working on various ways of looking into that at the cellular level and using microscopy and genetics.”
As expected from the initial testing, they are finding that gramillin is extremely toxic to some plants. They are also seeing a range of resistance within certain plant species.
“Within barley and corn, for example, we see a range of resistance all the way from extremely resistant to extremely susceptible,” notes Brauer. “That is really exciting to us because it means there is an opportunity to hone in on the genetics that are involved in that response.”
The project’s other goal is to examine the kinds of responses that gramillin produces in plants. “For instance, we see that gramillins can activate some of the responses that are associated with immune responses in plants. And some of the genes involved in gramillin responsiveness do influence fusarium resistance.”
The project includes a collaborative effort to map the genetic regions that play a part in gramillin resistance/susceptibility in barley. Barley researchers and breeders with AAFC and with Olds College of Agriculture and Technology in Alberta have been making crosses between a gramillin-resistant barley and a gramillin-sensitive barley in order to create mapping populations of barley lines. These collaborators are also genotyping the lines in these populations.
The role of Brauer’s lab in this effort was to figure out how to measure gramillin sensitivity in a reliable way so they can determine the gramillin responses across the hundreds of different genotypes in the mapping populations.
Then, using the genotype data and the gramillin response data for the lines, the researchers will be able to find the genes involved in gramillin resistance and develop molecular markers for those genes. Those markers can then be used by breeders to track the presence of gramillin resistance in their barley breeding materials.
The project team expects to complete the project and publish their results fairly soon.
Wider potential benefits
Developing markers for gramillin resistance is the first stage in a longer term goal of Brauer’s lab. “We want to be able to provide information about the different forms of fusarium resistance so breeders can track the ‘flavour’ of resistance they are working with in their breeding materials – so they will know if it is gramillin resistance or DON resistance or some other type of resistance,” she says.
With that information, breeders will be able to more accurately and effectively stack different forms of FHB resistance together in their barley varieties.
Achieving this goal will require similar research to identify more of the virulence factors in F. graminearum’s armoury and to understand the mechanisms and genes used by various hosts to resist these toxins.
According to Brauer, the gramillin research could also help towards tackling other diseases. She explains that the gramillins are examples of a large group of metabolites called cyclic lipopeptides. Cyclic lipopeptides are released by a lot of pathogenic microbes, including many different Fusarium species. “If we can understand how crops respond to the gramillins, it could have implications for other types of pathogens.”
Brauer really appreciates the funding support from producer groups for this gramillin research, but even more than that, she appreciates the continuing conversations with Canadian farmers about what they need from agricultural research. “I think of it as a partnership to help us understand how we can best serve farmers,” she notes.
“I have heard from farmers loud and clear that fusarium head blight is a serious problem and that they need solutions that are simple to deploy. So, I think that genetic resistance is an important target even though it is a very challenging target to go after. In recent years, we have seen some real progress in understanding FHB resistance, and we are really hoping to help move genetic resistance further forward.”
Collaborators on this project include: Flavio Capettini and Jennifer Zantinge at Olds College of Agriculture and Technology; and Ana Badea, James Tucker, Raja Khanal, and Barbara Blackwell at AAFC.