
Features
Multiple-group resistance in wild oat is challenging
Herbicide choices are becoming very small.
September 1, 2021 By Bruce Barker
 Farmers with wild oat resistant to Group 1+2 herbicides have few herbicide options.
Photo by Bruce Barker.
Farmers with wild oat resistant to Group 1+2 herbicides have few herbicide options.
Photo by Bruce Barker. Testing 1, 2, 8, 14, and 15. Those are the herbicide groups to which wild oats are resistant in Western Canada. In some crops, there are few – if any – choices for controlling a wild oat that has multiple resistance to those herbicide groups, especially Groups 1+2 biotypes.
“Herbicide resistance in wild oat is arguably the worst weed problem that Prairie growers face,” says Charles Geddes, weed scientist with Agriculture and Agri-Food Canada in Lethbridge, Alta. “With wild oat resistance to up to five herbicide modes-of-action in the same population, growers are in need of alternate solutions.”
Overall, in the last herbicide-resistant weed surveys across the Prairies – conducted from 2014 to 2017 by weed scientist Hugh Beckie when he was with AAFC Saskatoon – of 578 fields where wild oat seed was collected, 69 per cent had a resistant biotype, with Group 1 resistance in 62 per cent, 34 per cent with Group 2 resistance, and 27 per cent with Group 1+2 resistance.
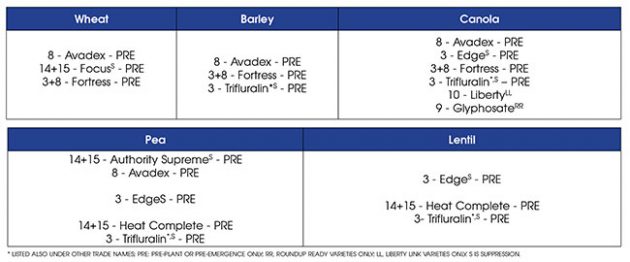
“Assuming a field has Group 1+2 resistant wild oat, in wheat, barley, pea and lentil, a grower only has pre-emergent herbicide options left. In canola, they would also have the option of Liberty and glyphosate in the herbicide resistance systems,” Geddes says.
Making herbicide selection even tougher is that Group 1+2+8 resistance has also been confirmed in all three Prairie provinces. This would remove triallate (Avadex and found in part of Fortress) from the list of effective herbicides. The trifluralin portion of Fortress may still provide suppression of wild oat.
Even worse, Group 1+2+8+14+15 wild oat resistance has been confirmed in Manitoba, which leave only Group 3 herbicides for wild oat suppression.
Target-site resistance versus metabolic resistance
Multiple types of herbicide resistance can occur, but they fit into two general categories: target-site resistance and non-target-site resistance. With target-site resistance, an herbicide binds to an enzyme target site to control the weed. When target-site resistance develops, it is like trying to fit a round peg into a square hole – it doesn’t fit so the herbicide doesn’t bind fully to the target site.
Target-site resistance develops as the result of random and naturally occurring mutations in a very low proportion of the wild oat population. Eventually, with increased selection pressure of the herbicide over the years, populations of the mutant biotype remain uncontrolled, multiply, and become the dominant biotype in the field. When this happens, the populations can survive high doses of the herbicide. Rotating or mixing herbicide groups can help slow the development of this type of resistance.
Metabolism-based resistance is one main type of non-target-site resistance. It occurs when the weed is able to break down or degrade the herbicide into less toxic metabolites before the herbicide reaches the target site. Low levels of resistance initially develop, but over time, the metabolic resistance builds to where a recommended herbicide dose does not control the weed. Herbicide rotation can be less effective in managing metabolic resistance after it has developed, because metabolic resistance can confer broad cross-resistance to multiple different herbicide modes of action – even ones that have never been used in the field.
Within Group 1, Beckie found there were differences in cross resistance across the three chemical families commonly referred to as “fops,” “dims” and “dens.” In his research in 2001, he found that wild oat resistance was found more in the “fops” than the “dims.” In the “dims,” clethodim had a very low level of resistant biotypes. However, continual use of the “dims” for wild oat control would select for resistant biotypes, either through target-site resistance or metabolic resistance.
In Group 2, there are six chemical families. Beckie found that the Group 2 families were more likely to have cross-resistance across all families, such as the imidazolinones, sulfonylurueas, and sulfonylamino-carbonytriazolinones.
“This cross-resistance in Group 2 families is likely because of the development of more metabolic resistance, but further research is required,” Geddes says.
For growers interested in knowing if they have weed resistance on their farm and what kind, several labs across the Prairies offer testing for $125 to $200 per sample and herbicide subgroup. These include the Saskatchewan Ministry of Agriculture’s Crop Protection Lab and Ag-Quest in Minto, Man.
In Alberta, Geddes’ Prairie Herbicide Resistance Research Lab is looking for novel herbicide-resistant weed biotypes. If undocumented resistance to an herbicide mode of action is suspected, growers and agronomists can send a sample for free testing. The lab is also accepting kochia samples for Group 4 (auxinic) herbicide resistance testing.
Geddes says, “Diagnostic testing can be an excellent investment in helping to understand herbicide resistance on your farm, and how to manage it.”
World’s first metabolism-based glyphosate resistance
At the Australian Herbicide Resistance Initiative (AHRI) at the University of Western Australia (where Beckie now works), researchers have identified the world’s first metabolism-based glyphosate resistance. A population of barnyard grass was confirmed with glyphosate resistance, and during the testing the researchers noticed the level of resistance was influenced by temperature, indicating that the mechanism might be metabolism-based.
The field was a horticulture field under drip irrigation, so tillage could not be used to control weeds. The field was aerially sprayed with five to six
litres per hectare of glyphosate, five to six times per wet season for 12 to 15 years in a row. The researchers used a combination of genetic testing and rice calli tissue culturing to confirm the metabolic resistance.
While this is the first case of metabolism-based glyphosate resistance, it shows that evolution continues, and won’t likely be the last. This highlights the need for growers to use all their tools to manage herbicide resistance.