
Features
Agronomy
Fertility and Nutrients
Glowing recommendations
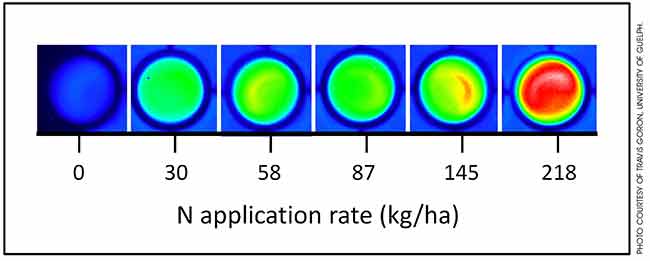
A biosensor called GlnLux can detect differences in nitrogen levels that could help corn growers make decisions on sidedressing nitrogen at the six-leaf stage. Photo courtesy of Travis Goron, University of Guelph.
A special bacterium that glows when nitrogen is present shows great promise as a low-cost, rapid and accurate biosensor for assessing nitrogen levels.
“The idea is to help crop growers decide whether or not they should sidedress with nitrogen and, if so, by how much,” says Manish Raizada, an associate professor of plant agriculture at the University of Guelph.
For corn crops, splitting nitrogen fertilizer into two applications – with one at planting and the other as a sidedress – is less risky than the more common approach of a single application at planting.
“When seedlings are really small, they don’t have much nitrogen demand. On top of that, in the spring, nitrogen is also coming from decomposing soil organic matter as the temperature warms. The estimates are that, on average, only about 50 per cent of the nitrogen [fertilizer applied at planting time] is taken up by the corn plants. As a result, a lot of the nitrogen is lost [through leaching and gaseous emissions],” Raizada explains. Those losses represent a waste of money for the grower and may contribute to groundwater pollution and greenhouse
gas emissions.
Sidedressing time is usually about the six-leaf stage (V6). Compared to planting time, V6 timing has a lower risk of nitrogen loss because it’s closer to the time of the corn crop’s maximum nitrogen uptake, which occurs between about V9 and just before tasseling. Raizada recognizes that applying the second dose later than V6 could be beneficial, but such applications would require highboy equipment, which only some growers have.
Pre-sidedress soil nitrogen tests can be used to help figure out sidedress nitrogen rates, but soil testing has a couple of disadvantages. One is that it can be expensive. Raizada notes, “You need to do a lot of soil sampling both spatially and at different times in the season [because nitrogen levels vary due to factors like soil texture and weather conditions]. A soil test costs about $10 per sample here, and in developing countries it often costs about $20 per sample, which is prohibitively expensive for farmers in those countries.”
In addition, a soil test doesn’t tell you how much nitrogen is actually available to the plant in the short term, which is important for determining nitrogen fertilizer needs. He explains, “A lot of the nitrogen in the soil is not immediately available to plants [because it is tied up in living organisms, decaying organic matter or bound to soil particles]. What we really need to know is how much nitrogen a plant is able to take up.”
In contrast, the biosensor test developed at Raizada’s lab costs only about $1 per sample, and it tells you how much nitrogen the plant is actually taking up.
How it works
“This is a very simple biosensor. It is based on the concept of an auxotroph. An auxotroph is a cell strain that has a mutation in the genome that prevents it from growing unless you add a missing nutrient. In this case, we’re using a harmless E. coli strain that has a mutation in a gene that makes the amino acid glutamine. So the cell cannot live without added glutamine,” Raizada says. “Now, we have inserted a gene into this microbe so that it gives off some light when it’s alive.”
So, when the bacterial cells have a little bit of glutamine in their environment, they can stay alive, divide and grow. Every time a cell multiplies, its light output doubles. As the amount of glutamine increases, the cell population increases and the light output increases. That light output can be quickly and easily measured with a machine.
The researchers have named the biosensor “GlnLux” – “Gln” for glutamine, and “lux”, which is the Latin word for light and a unit of measure for light emissions.
Why glutamine? “When the plant takes up nitrogen in its roots, it almost immediately converts that into different amino acids, and glutamine is one of the most important amino acids,” Raizada explains.
“Then that glutamine is transported from the root to leaves. Glutamine is used as a primary building block for plant tissues – to build other amino acids, and also chlorophyll, DNA and anything else that requires nitrogen. But glutamine is also the primary transport form of nitrogen in corn.”
As the main transport form, glutamine is coursing through the plant’s vascular tissues, such as leaf veins. By taking a sample from a leaf vein, for example, you can measure the current level of free glutamine, rather than the glutamine that has been bound up in various forms in the plant over the previous weeks.
Optimizing GlnLux
Michael Tessaro, a masters student in Raizada’s lab, built and tested the biosensor. His lab tests showed that GlnLux is as sensitive and accurate as high-performance chemical tests for glutamine, plus GlnLux is cheaper, faster and easier to use than those chemical methods.
More recently, Travis Goron, one of Raizada’s PhD students, has been optimizing the GlnLux sampling and testing procedures, and testing whether the GlnLux technology will work under greenhouse and field conditions.
Goron has developed a fairly simple set of procedures. Using a leaf puncher, which is similar to a hole puncher for paper, you punch a vein at the tip of a young, growing leaf. Only a single little leaf punch is needed per plant because the biosensor test is extraordinarily sensitive. Each leaf punch is collected in its own tube. You put the tube in a well in a sample tray; Raizada’s lab usually uses trays with 96 wells.
Next, you grind each leaf punch; the researchers have developed a mechanical method of grinding hundreds of samples at a time. Then you dilute each sample 1000 times, producing an extract that contains free glutamine. You add the GlnLux cells to each sample and let the samples sit for about three hours, allowing the glutamine to cause the dormant GlnLux cells to come alive, grow, divide and glow.
Then, you put the samples into a machine. In two minutes, that machine reads the amount of light being emitted from each sample, allowing the researchers to determine the glutamine levels in 96 plants. The capital cost of the machine is about $15,000 to $20,000. It has the potential to read 400 samples at a time.
In his proof-of-concept trials, Goron first tested about 3000 leaf punches from corn plants grown in clay rock in a greenhouse, comparing the effects of different amounts of added nitrogen.
Unlike soil, the clay rock has no background levels of nitrogen, so this approach provided a very accurate way to evaluate the effects of added nitrogen on GlnLux light output.
For the greenhouse samples, the light output was directly proportional to the amount of added nitrogen.
Goron tested thousands of leaf punches from corn grown at two Ontario field sites in 2014 and 2015. These tests were done in collaboration with Bill Deen, an associate professor in plant agriculture at the University of Guelph, and Greg Stewart, who was the corn specialist with the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA).
Deen and Stewart allowed Goron to take leaf punches at V3, V6, V9 and V12 from the corn plants in their nitrogen rate trials.
For comparison, Deen and Stewart tested other ways of determining in-crop nitrogen requirements on their plots, including pre-sidedress soil tests and commercial plant-based approaches such as GreenSeeker, GreenIndex and SPAD.
As well, Goron tested leaf punches from finger millet. “Finger millet is an important African and South Asian crop grown by subsistence farmers. Our lab was growing finger millet for other studies, so we decided to test it,” Raizada says.
Like the greenhouse findings, the light output was strongly correlated with the nitrogen fertilizer rate for the field samples. Those strong correlations started at V6 and continued for V9 and V12.
The V6 correlations were much stronger for GlnLux than for the other plant-based approaches. “The other plant-based methods are based on how much chlorophyll the plant is producing because nitrogen is a building block for chlorophyll. But early in the growing season, chlorophyll is not limited; only when nitrogen gets really limited is the plant not making chlorophyll. Also chlorophyll accumulates, so the amount of chlorophyll in a leaf doesn’t necessarily tell you if the plant is experiencing a nitrogen deficiency in a particular month,” Raizada explains.
“In contrast, the free levels of glutamine are very dynamic. In fact, we think if a plant is limited for nitrogen even on a particular day, you might see it in our leaf punches. In any case, our test is more dynamic, it has much better resolution and, most importantly, it works in the early season. Some of the plant-based tests work really well in the late season, which is too late in terms of sidedress recommendations.”
Perhaps the most exciting finding from Goron is that the early-season GlnLux reading predicts the final grain yield. Raizada says, “Ultimately, that is what a grower wants to know: early in the season, if I add this much nitrogen, here is what my yield is going to be. Across two seasons of corn and finger millet – two very different cereal crops – the correlation between the early-season GlnLux reading and grain yield is highly significant. That result is so amazing that we have submitted and have a provisional U.S. patent on just that aspect of the work.”
A mail-in kit – and more
Next, the researchers are hoping to develop a practical, low-cost mail-in kit for growers.
“We estimate the cost of the test, including labour, is $1 per sample. So, rather than do 100 soil samples at $1000, a grower could do 1000 leaf punch samples for $1000, or 100 samples for $100,” Raizada says.
The idea would be that, for about $100, a grower would receive a kit including leaf punch tubes, sampling instructions, a way to record the sample locations for each tube, an overnight courier envelope for sending the samples to a diagnostic lab and a password for accessing the sample results and nitrogen rate recommendations on the diagnostic lab’s website.
The current challenge is to make the sample handling procedures more convenient for growers. Then Goron will work with Deen to develop an algorithm, or perhaps several algorithms, to convert the GlnLux readings into nitrogen rate recommendations, taking into account factors like soil texture, fertilizer costs, corn prices, precipitation amounts, and so on.
Also, like any other new technology, the kit will need to go through a beta testing phase to make sure the sampling, handling and testing procedures and the algorithms are robust. Raizada has already begun discussions with grower associations in Canada and the United States regarding the possibility of collecting leaf punch samples from many locations for this phase.
It’s hard to say how long it will take to finalize the mail-in kit, but Raizada notes, “What I can say today is, at the very least, we have a great tool for researchers.” He sees many immediate applications for GlnLux in which researchers could use this cheap, rapid technology, rather than doing more costly analyses, like soil and plant tissue testing, or waiting for crop yield results.
For example, a crop breeder who wants to screen breeding lines for nitrogen use efficiency would have a low-cost option. Other possible users include fertilizer companies wanting to evaluate the effects of their new fertilizer technologies, or microbial companies wanting to assess how their plant-growth-promoting microbial products affect nitrogen levels in various plant tissues at various crop stages.
“GlnLux is already opening up interesting possibilities,” Raizada says.
Funding for the GlnLux research is from OMAFRA, the Grain Farmers of Ontario and, most recently, the Natural Sciences and Engineering Research Council of Canada.
May 31, 2016 By Carolyn King